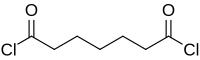
Pimeloyl chloride
| |
| Names | |
|---|---|
| Preferred IUPAC name
Heptanedioyl dichloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.005.056 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H10Cl2O2 | |
| Molar mass | 197.06 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H315, H318, H335 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pimeloyl chloride is a di-acyl chloride. It is used as a reagent in organic synthesis.
Synthesis[edit]
Pimeloyl chloride can be synthesized from pimelic acid in thionyl chloride.[1]
References[edit]
- ^ US 2014256775, CHEN LIN [US]; CHEN XIAOJIANG [US]; WU YONGQING[US]; GAI DAHAI [US], "NOVEL TRANSCRIPTION FACTOR MODULATORS"